–News Direct–
By Jeremy Golden, Benzinga
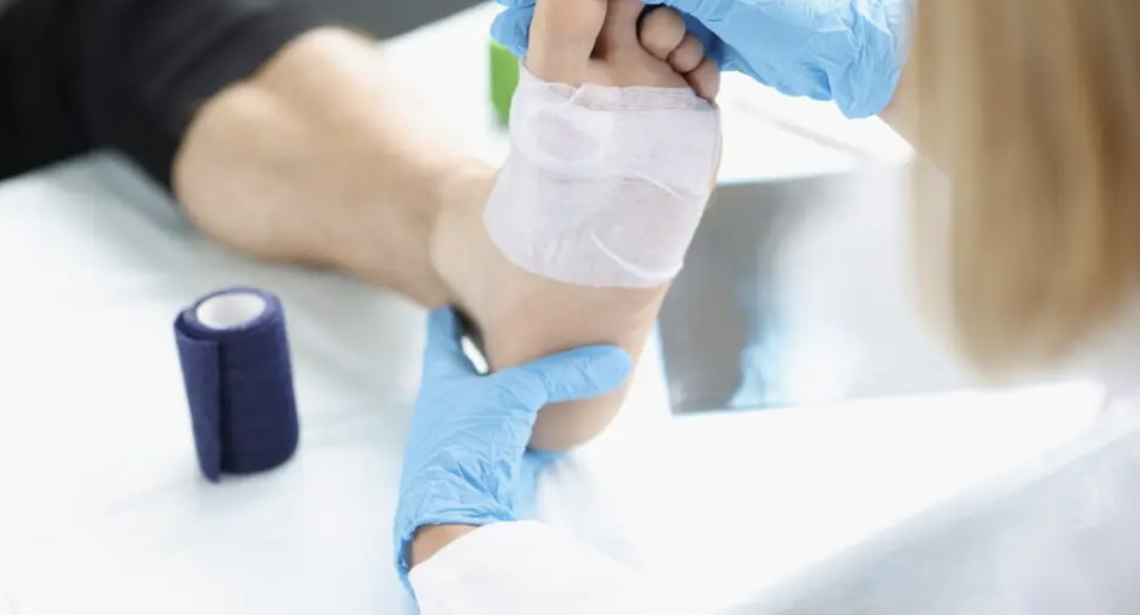
IVRN has completed a 510(k) submission for its SkinDiscTM Lite and SkinDiscTM regenerative medicine technology. The company says that what differentiates this technology from other regenerative products is the fact that it only requires one application to achieve healing in four to nine weeks and comes packaged as a disposable kit.
An aging U.S. population with increasingly complex medical diagnoses has doctors and clinicians under pressure to diagnose and treat patients while restoring their quality of life. With the goal of doing just that, a life science and biotech incubator company is advancing a wound-healing product.
Focused on advancing new technologies in areas of unmet need across multiple indications, Innoveren Scientific Inc.
This is a significant advancement when compared to current treatment approaches that require weekly applications and can take 12-20 weeks to heal. Generally, people have to apply treatment products every three days before they see any impact. With SkinDisc, only one application is needed.
SkinDiscTM was acquired by Innoveren Scientific in December of 2022. A breakthrough stem cell therapy technology, SkinDiscTM has positioned the company to combat the rising prevalence of acute, chronic and surgical wounds with an effective and affordable treatment. As outlined in its submission, SkinDiscTM combines stem cells from the patient and several other molecular components to stimulate tissue regeneration, which results in rapid growth of the host tissue.
To best serve those who can benefit from this proprietary technology, Innoveren Scientific plans to work with surgeons, podiatrists and physicians to treat patients with severe trauma-based wounds and non-healing chronic wounds, which affected nearly 16.3% of Medicare beneficiaries in 2019, according to a recent study by the University of Pittsburgh Medical Center. Those numbers are only expected to continue on an upward trajectory.
Innoveren's recent submission to the U.S. Food & Drug Administration (FDA) is primarily focused on chronic non-healing wounds and burns, an area of medicine where current treatment options have stagnated. With patented or under-patented components, SkinDiscTM has treated over 500 patients to date. Innoveren reports that early clinical outcomes of those who used SkinDiscTM demonstrated significant results relative to current treatment options in wound healing and limb salvage.
Innoveren Scientific expects to receive a response from the FDA by mid-May 2024 for both SkinDiscTM and SkinDiscTM Lite.
This 510(k) submission is one of three the company has in its pipeline utilizing the body's own cells to achieve successful recovery of the patient. In the coming months, Innoveren plans to submit an application to the FDA for its SkinDiscTM Ultra technology, an iteration that incorporates Bone Marrow Aspirate (BMA) and would only be available for use in operating rooms and limb salvage procedures; specifically in cases of severe trauma, infections or diabetic foot ulcers.
In addition to SkinDiscTM, the company also has BreatheEasy, a 510(k) De Novo pathway medical device that helps patients struggling with chronic obstructive pulmonary disease (COPD), as well as other closely related diseases such as chronic bronchitis and emphysema.
Featured photo by megaflopp on Shutterstock.
Benzinga is a leading financial media and data provider, known for delivering accurate, timely, and actionable financial information to empower investors and traders.
This post contains sponsored content. This content is for informational purposes only and is not intended to be investing advice.
Contact Details
Benzinga
+1 877-440-9464
Company Website
View source version on newsdirect.com: https://newsdirect.com/news/innoveren-scientifics-otcmkts-ivrn-510-k-submission-sparks-a-new-hope-for-patients-living-with-chronic-non-healing-wounds-944089317
Benzinga
COMTEX_448891123/2655/2024-03-07T08:36:10
Disclaimer: The views, suggestions, and opinions expressed here are the sole responsibility of the experts. No State Today USA journalist was involved in the writing and production of this article.